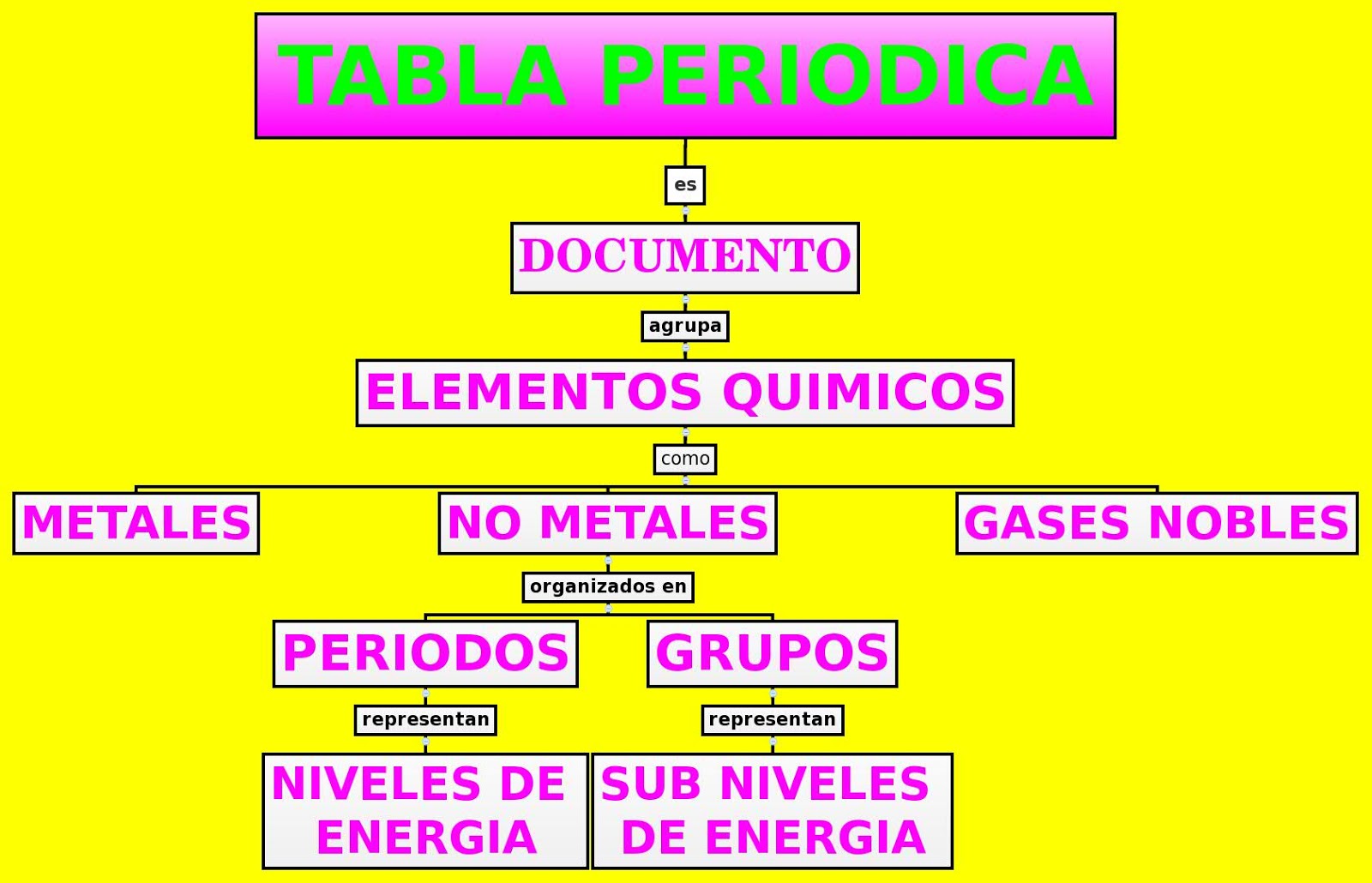
Unlocking the Secrets of the Esquema de la Tabla Periodica
Imagine trying to understand a vast library of books without any system of organization. It would be overwhelming and nearly impossible to find the information you need. In the world of chemistry, the "esquema de la tabla periodica" – or as it's known in English, the periodic table – serves as that essential organizing principle. It's not just a chart; it's a map that reveals the intricate relationships between all the known elements that make up our universe.
From the air we breathe to the technology we rely on, everything around us is built from these fundamental building blocks. The periodic table, with its seemingly simple arrangement of rows and columns, holds the key to understanding the properties, behaviors, and potential of each element. It's a testament to the power of human observation, experimentation, and the pursuit of patterns within the seemingly chaotic natural world.
The journey to create this elegant framework began centuries ago with alchemists striving to transmute metals. As scientists delved deeper into the nature of matter, they discovered more and more elements, leading to a pressing need for classification. This need gave birth to early attempts at organizing elements based on their properties, but it was Dmitri Mendeleev's groundbreaking work in the late 19th century that truly revolutionized our understanding.
Mendeleev's periodic table, arranged by atomic weight and grouped by similar chemical properties, wasn't merely a list; it was a prediction machine. He boldly left gaps in his table, predicting the existence and properties of elements yet to be discovered. The subsequent discovery of elements like gallium, scandium, and germanium, which perfectly matched Mendeleev's predictions, solidified the table's power and cemented its place as a cornerstone of modern chemistry.
Today, the periodic table continues to evolve as we probe the furthest reaches of nuclear physics, synthesizing new elements and pushing the boundaries of our understanding. Yet, its fundamental structure remains a testament to the enduring genius of Mendeleev's vision. The table is an indispensable tool for students, chemists, and researchers, serving as a constant source of inspiration and a guide to unraveling the complexities of the material world.
Understanding the periodic table is about much more than memorizing symbols and atomic numbers. It's about grasping the fundamental principles that govern the interactions of elements, the patterns that emerge from their arrangement, and the predictive power this knowledge bestows. It's about appreciating the beauty of a system that links together everything from the smallest atom to the largest star, revealing the interconnectedness of our universe at its most fundamental level.
Advantages and Disadvantages of Using the Periodic Table
| Advantages | Disadvantages |
|---|---|
| Provides a systematic organization of elements. | Can be overwhelming for beginners due to its complexity. |
| Predicts the properties of elements based on their position. | Doesn't provide detailed information on isotopes or nuclear properties. |
| Facilitates the study of chemical reactions and bonding. | May not fully capture the nuances of element behavior in all conditions. |
Common Questions about the Periodic Table
1. What is the significance of the periods (rows) in the periodic table?
The periods represent the number of electron shells an atom of that element possesses. As you move down a group, the atomic radius generally increases due to the addition of another electron shell.
2. What do the groups (columns) in the periodic table tell us about the elements within them?
Elements within the same group share similar chemical properties due to having the same number of valence electrons—those in the outermost shell involved in bonding.
3. How can the periodic table help me understand chemical bonding?
The periodic table reveals which elements are likely to gain, lose, or share electrons to achieve a stable electron configuration, thus predicting the types of bonds they form.
4. What is the difference between metals, nonmetals, and metalloids on the periodic table?
The periodic table is broadly divided, with metals typically on the left, nonmetals on the right, and metalloids exhibiting properties of both along a diagonal line.
5. Why are some elements on the periodic table synthetic?
Elements beyond uranium (atomic number 92) are not found naturally and are created in laboratories through nuclear reactions.
6. How has the periodic table changed over time?
New elements have been discovered and added, and our understanding of atomic structure has led to refinements in the table's organization.
7. What are some resources for further exploring the periodic table?
Numerous websites, textbooks, and interactive apps offer in-depth information and engaging ways to learn about the elements and their relationships.
8. How does the periodic table connect to other scientific disciplines?
Its principles are applied in fields like materials science, astronomy, medicine, and environmental science, illustrating its broad impact.
Conclusion
The "esquema de la tabla periodica," or periodic table, stands as a monument to scientific inquiry and the human desire to understand the world around us. It's a powerful tool that transcends language barriers and unites minds across disciplines in a shared quest for knowledge. From unraveling the mysteries of chemical reactions to designing new materials with remarkable properties, the periodic table is an indispensable guide on our journey of discovery. By delving into its intricacies, we gain a deeper appreciation for the elegance and order woven into the fabric of our universe. Let this exploration be a starting point for you to continue uncovering the wonders hidden within the periodic table and embrace its power as a key to unlock the secrets of matter.
Jessica tarlov height and age unveiling the facts
Craving the highlights what you missed in benficas jogo ontem
The intriguing world of facial moles significado de lunar en la cara