Unlocking Chemistry Secrets: Exploring the Tabla Periodica Con Las Valencias
Ever wondered how chemists predict the behavior of elements and form compounds? The answer lies within a fundamental tool: the periodic table, or as we say in Spanish, the "tabla periodica." This ingeniously arranged chart, with its rows and columns, is much more than just a list of elements—it's a roadmap to understanding the building blocks of our universe.
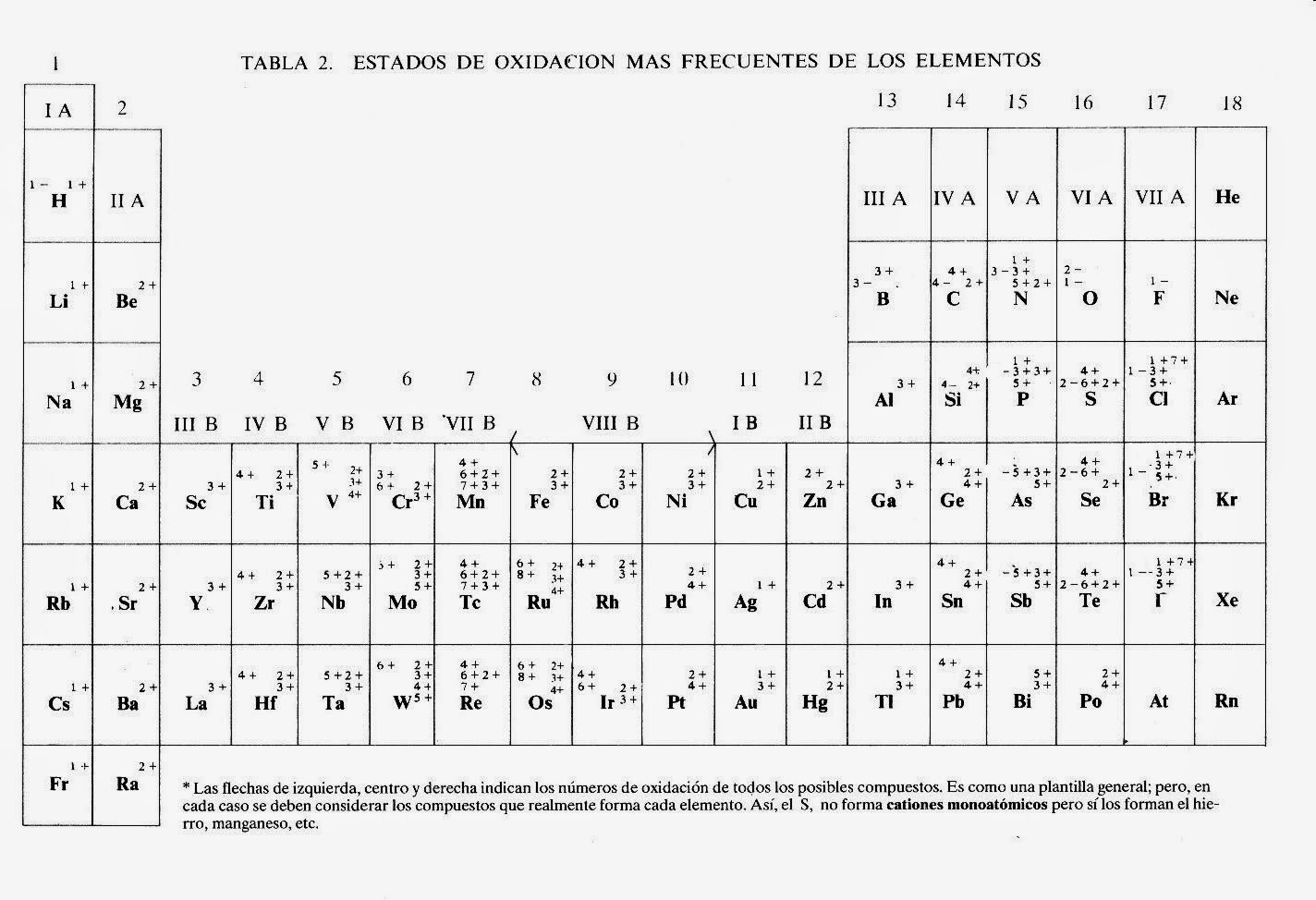
At the heart of the periodic table's predictive power lies the concept of "valencia," which translates to "valence" in English. Valence refers to the combining power of an element, essentially dictating how many bonds an atom can form with other atoms. Think of it as the social life of an element - some are eager to bond, while others prefer to be solo.
The history of the periodic table is a fascinating journey of scientific discovery. In the mid-19th century, Dmitri Mendeleev, a Russian chemist, noticed patterns in the properties of known elements. His groundbreaking insight was to arrange elements by increasing atomic weight, revealing recurring trends or periodicity in their properties. Mendeleev's genius wasn't just in organizing the known; he boldly left gaps, predicting the existence and properties of yet-to-be-discovered elements.
Fast forward to today, and Mendeleev's periodic table has become an iconic symbol of scientific order and insight. It's an indispensable tool for chemists, students, and anyone curious about the elements that make up our world.
Understanding valence, a key feature represented within the periodic table, is crucial. An element's position on the table hints at its valence. For instance, elements in the same vertical column, known as a group, often share similar valences. Take Group 1, the alkali metals – lithium, sodium, potassium – they all have a valence of +1, meaning they readily lose one electron to form a bond. This predictability is invaluable in chemistry!
Advantages and Disadvantages of the Periodic Table
While the periodic table is a powerful tool, it's not without its limitations. Let's delve into its pros and cons:
| Advantages | Disadvantages |
|---|---|
| Predicts element properties | Doesn't show the exact value of valence for all elements, as it can vary depending on the chemical context. |
| Organizes elements systematically | Placement of some elements, like hydrogen, can be debated. |
| Facilitates the study of chemistry | Doesn't provide information about isotopes. |
Best Practices for Utilizing the Periodic Table
- Understand the layout: Familiarize yourself with groups, periods, and what information each cell provides.
- Focus on trends: Notice how properties like atomic radius and electronegativity change across periods and down groups.
- Use it for predictions: Use the table to hypothesize about an element's reactivity based on its position.
- Explore beyond the basics: Dive into the rich history and the stories behind the discovery of each element.
- Don't be afraid to ask questions: The periodic table is a gateway to a vast and exciting field - curiosity is key!
The periodic table, with its intricate arrangement of elements and the embedded concept of valence, is much more than a chart—it's a testament to the power of human observation, organization, and the quest to understand the universe at its most fundamental level. Whether you're a budding chemist or simply curious about the elements around you, the periodic table offers a fascinating journey of discovery.
Isles homes living a modern approach to island life
Unlocking the power of behr gloss white paint
Remember elwood city a nostalgic dive into pbs kids arthur episodes